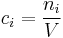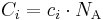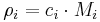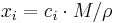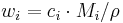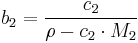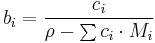Molar concentration
In chemistry, the molar concentration, 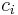 is defined as the amount of a constituent
is defined as the amount of a constituent 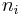 divided by the volume of the mixture
divided by the volume of the mixture 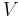 [1]:
[1]:
It is also called molarity, amount-of-substance concentration, amount concentration, substance concentration, or simply concentration. The volume 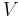 in the definition
in the definition 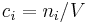 refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
Contents |
Units
The SI-unit is mol/m3. However, more commonly the unit mol/L is used. A solution of concentration 1 mol/L is also denoted as "1 molar" (1 M).
- 1 mol/L = 1 mol/dm3 = 1 mol dm−3 = 1 M = 1000 mol/m3.
An SI prefix is often used to denote concentrations. Commonly used units are listed in the table below:
| Name | Abbreviation | Concentration | Concentration (SI-unit) |
|---|---|---|---|
| millimolar | mM | 10−3 mol/dm3 | 100 mol/m3 |
| micromolar | μM | 10−6 mol/dm3 | 10−3 mol/m3 |
| nanomolar | nM | 10−9 mol/dm3 | 10−6 mol/m3 |
| picomolar | pM | 10−12 mol/dm3 | 10−9 mol/m3 |
| femtomolar | fM | 10−15 mol/dm3 | 10−12 mol/m3 |
| attomolar | aM | 10−18 mol/dm3 | 10−15 mol/m3 |
| zeptomolar | zM | 10−21 mol/dm3 | 10−18 mol/m3 |
| yoctomolar | yM[2] | 10−24 mol/dm3 (1 molecule per 1.6 L) |
10−21 mol/m3 |
Related Quantities
Number concentration
The conversion to number concentration 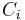 is given by:
is given by:
where 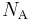 is the Avogadro constant, approximately 6.022×1023 mol−1.
is the Avogadro constant, approximately 6.022×1023 mol−1.
Mass concentration
The conversion to mass concentration 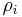 is given by:
is given by:
where 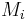 is the molar mass of constituent
is the molar mass of constituent 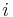 .
.
Mole fraction
The conversion to mole fraction 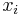 is given by:
is given by:
where 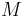 is the average molar mass of the solution and
is the average molar mass of the solution and 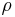 is the density of the solution.
is the density of the solution.
Mass fraction
The conversion to mass fraction 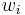 is given by:
is given by:
Molality
The conversion to molality (for binary mixtures) is:
where the solute is assigned the subscript 2.
For solutions with more than one solute, the conversion is:
Properties
Dependence on volume
Molar concentration depends on the variation of the volume of the solution due mainly to thermal expansion.
Examples
Example 1: Consider 11.6 g of NaCl dissolved in 100 g of water. The final mass concentration 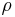 (NaCl) will be:
(NaCl) will be:
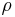 (NaCl) = 11.6 g / (11.6 g + 100 g) = 0.104 g/g = 10.4 %
(NaCl) = 11.6 g / (11.6 g + 100 g) = 0.104 g/g = 10.4 %
The density of such a solution is 1.07 g/mL, thus its volume will be:
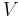 = (11.6 g + 100 g) / (1.07 g/mL) = 104.3 mL
= (11.6 g + 100 g) / (1.07 g/mL) = 104.3 mL
The molar concentration of NaCl in the solution is therefore:
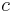 (NaCl) = 11.6 g / (58 g/mol * 104.3 mL) = 0.00192 mol/mL = 1.92 mol/L
(NaCl) = 11.6 g / (58 g/mol * 104.3 mL) = 0.00192 mol/mL = 1.92 mol/L
Here, 58 g/mol is the molar mass of NaCl.
Example 2: Another typical task in chemistry is the preparation of 100 mL (= 0.1 L) of a 2 mol/L solution of NaCl in water. The mass of salt needed is:
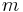 (NaCl) = 2 mol/L * 0.1 L * 58 g/mol = 11.6 g
(NaCl) = 2 mol/L * 0.1 L * 58 g/mol = 11.6 g
To create the solution, 11.6 g NaCl are placed in a volumetric flask, dissolved in some water, then followed by the addition of more water until the total volume reaches 100 mL.
Example 3: The density of water is approximately 1000 g/L and its molar mass is 18.02 g/mol. Therefore, the molar concentration of water is:
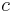 (H2O) = 1000 g/L / (18.02 g/mol) = 55.5 mol/L
(H2O) = 1000 g/L / (18.02 g/mol) = 55.5 mol/L
Likewise, the concentration of solid hydrogen (molar mass = 2.02 g/mol) is:
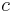 (H2) = 88 g/L / (2.02 g/mol) = 43.7 mol/L
(H2) = 88 g/L / (2.02 g/mol) = 43.7 mol/L
The concentration of pure osmium tetroxide (molar mass = 254.23 g/mol) is:
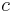 (OsO4) = 5.1 kg/L / (254.23 g/mol) = 20.1 mol/L.
(OsO4) = 5.1 kg/L / (254.23 g/mol) = 20.1 mol/L.
Example 4: Proteins in bacteria, such as E. coli, usually occur at about 60 copies, and the volume of a bacterium is about 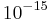 L. Thus, the number concentration
L. Thus, the number concentration 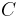 is:
is:
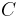 = 60 / (10−15 L)= 6×1016 L−1
= 60 / (10−15 L)= 6×1016 L−1
The molar concentration is:
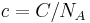 = 6×1016 L−1 / (6×1023 mol−1) = 10−7 mol/L = 100 nmol/L
= 6×1016 L−1 / (6×1023 mol−1) = 10−7 mol/L = 100 nmol/L
If the concentration refers to original chemical formula in solution, the molar concentration is sometimes called formal concentration. For example, if a sodium carbonate solution has a formal concentration of 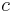 (Na2CO3) = 1 mol/L, the molar concentrations are
(Na2CO3) = 1 mol/L, the molar concentrations are 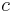 (Na+) = 2 mol/L and
(Na+) = 2 mol/L and 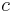 (CO32-) = 1 mol/L because the salt dissociates into these ions.
(CO32-) = 1 mol/L because the salt dissociates into these ions.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amount concentration, c".
- ^ David Bradley. "How low can you go? The Y to Y". http://www.sciencebase.com/yocto.html.
External links
- Molar Solution Concentration Calculator
- Experiment to determine the molar concentration of vinegar by titration
|
||||||||||||||